- Vitalspan- The Healthspan x Technology Enabler
- Posts
- Cell Therapy Restores Insulin Production in 83% of Type 1 Diabetics
Cell Therapy Restores Insulin Production in 83% of Type 1 Diabetics
Patients achieved insulin independence after one year
[A groundbreaking stem cell therapy called zimislecel has shown remarkable success in treating Type 1 diabetes, with 10 out of 12 patients achieving insulin independence after one year of treatment in a clinical trial.]
83% SUCCESS RATE
Patients achieved insulin independence after one year
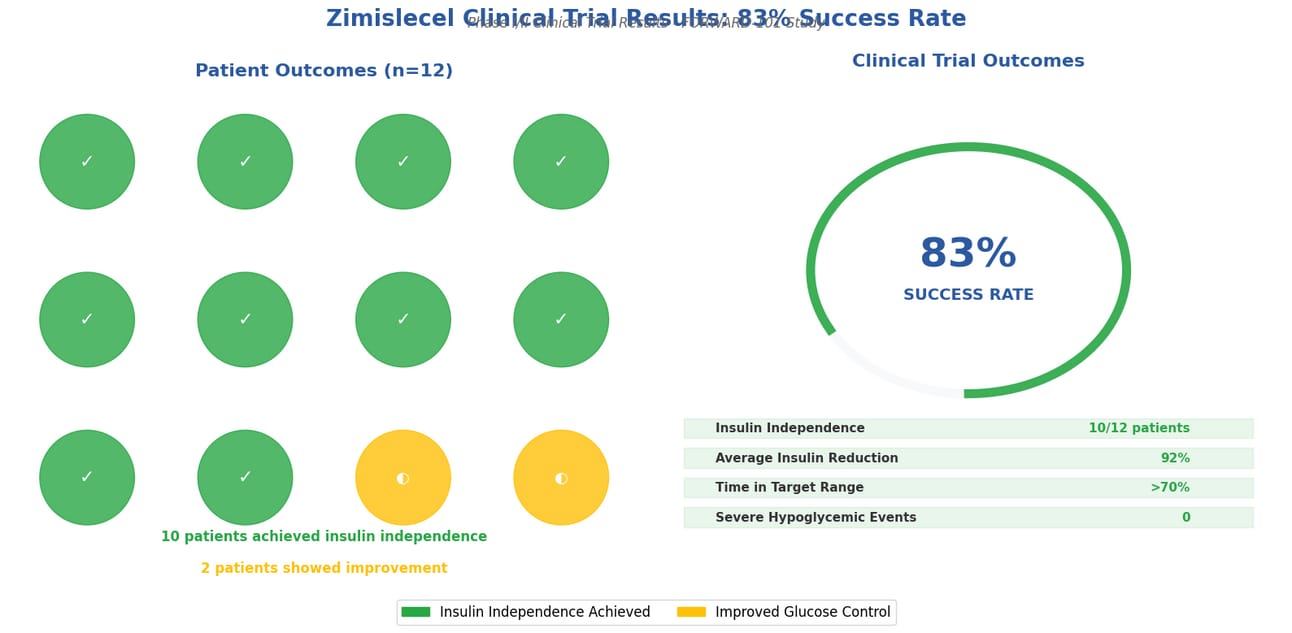
Clinical Trial Results: 10 out of 12 patients achieved insulin independence
The Game-Changing Discovery
Imagine never having to inject insulin again. For millions of people living with Type 1 diabetes, this sounds like a distant dream. But what if I told you that dream might be closer than you think?
Recent results from a clinical trial show that zimislecel, an experimental cell therapy for Type 1 diabetes, has demonstrated promise to restore insulin production, with 83% of patients stopping insulin injections after a year.
This breakthrough represents more than just numbers on a chart. It's about real people getting their lives back. Think about it: no more daily injections, no more constant blood sugar monitoring, no more fear of dangerous sugar crashes.
Understanding Type 1 Diabetes
Type 1 diabetes happens when your immune system mistakenly attacks the insulin-making cells in your pancreas. It's like having security guards that turn against the very building they're supposed to protect.
Before Treatment:
Daily insulin injections
Blood sugar crashes
Constant monitoring
Long-term complications
After Cell Therapy:
Natural insulin production
Stable blood sugar
Reduced monitoring
Better quality of life
How Does This Revolutionary Treatment Work?
The treatment, developed by Vertex Pharmaceuticals, uses a clever approach. Scientists take stem cells from donors and transform them into insulin-producing cells, just like the ones your pancreas should make naturally.
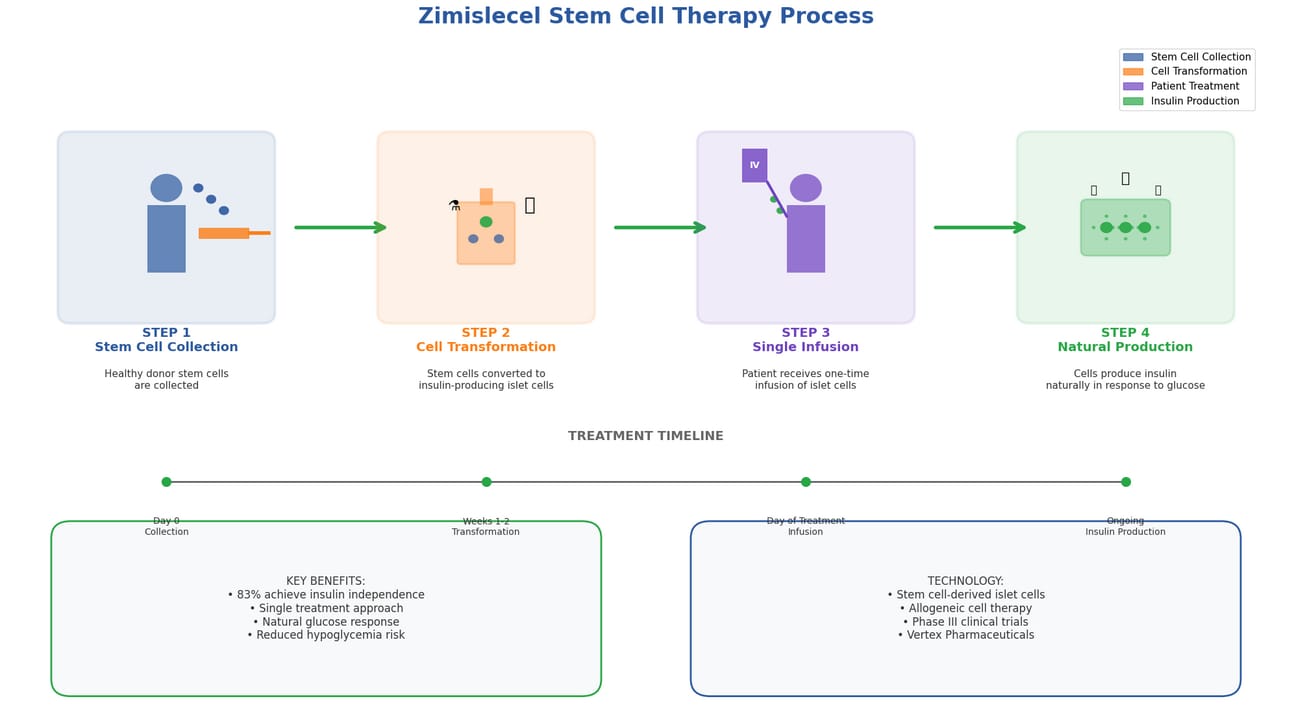
Complete zimislecel treatment process from stem cell collection to insulin production
The Four-Step Process:
Step 1: Stem Cell Collection Scientists collect stem cells from healthy donors
Step 2: Cell Transformation Stem cells are converted into insulin-producing islet cells
Step 3: Single Infusion Patient receives one infusion of these new cells
Step 4: Natural Production Cells begin producing insulin naturally
Think of it like giving your pancreas a team of skilled replacement workers. These new cells can sense when your blood sugar rises and respond by making just the right amount of insulin. It's like having a smart thermostat for your blood sugar levels.
"As I think about my patients and the unmet need in the type 1 diabetes community, the results we've seen so far for restoring endogenous insulin secretion with a stem cell-derived islet therapy bring me hope and confidence for a transformative treatment option."
- Dr. Michael R. Rickels, University of Pennsylvania
The Remarkable Results
The numbers from this study are nothing short of amazing. But what do they really mean for real people?
Key Study Results: ✓ All 12 patients who received a full dose improved their blood glucose control ✓ 10 out of 12 participants (83%) achieved insulin independence after one year ✓ 92% average reduction in insulin use ✓ All participants were free from severe hypoglycemic events ✓ Glycated hemoglobin levels below 7% ✓ Over 70% time in target glucose range
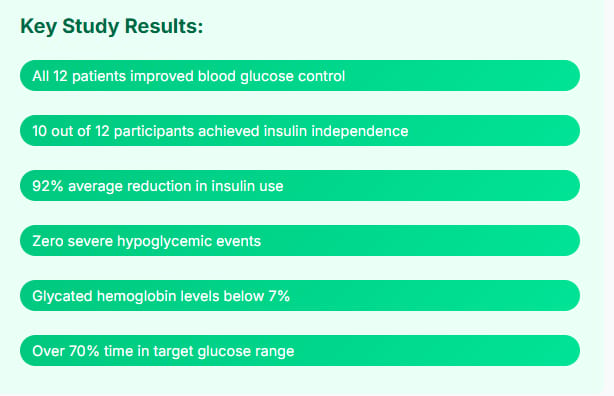
What makes these results even more impressive is their consistency. Every single patient in the study showed improvement. That's not something you see often in medical research.
Safety First: Is This Treatment Safe?
You might be wondering: "This sounds too good to be true. What's the catch?" It's a fair question, and safety is always the top priority in medical treatments.
The treatment proved safe and effective, with all participants demonstrating engraftment and insulin production. No serious adverse events were found. However, there's an important detail to understand.
Important Safety Considerations: Patients receiving this treatment need to take immunosuppressive medications to prevent their immune system from rejecting the transplanted cells. This is similar to what organ transplant patients take, and it requires careful monitoring by healthcare providers.
Think of immunosuppressive drugs as peacekeepers. They help prevent your immune system from attacking the new insulin-producing cells. While this adds complexity to the treatment, the benefits for many patients far outweigh the risks.
Who Can Benefit From This Treatment?
This isn't a treatment for everyone with Type 1 diabetes. The current focus is on a specific group of patients who face the most dangerous complications.
Zimislecel is aimed at patients diagnosed with Type 1 diabetes with impaired hypoglycemic awareness and severe hypoglycemia. These are people who can't feel when their blood sugar drops dangerously low, making them vulnerable to life-threatening episodes.
Why start with this group? Because for these patients, the risk of severe complications from their diabetes often outweighs the risks of immunosuppressive therapy. It's like choosing between two difficult paths, but one leads to a much better destination.
The Science Behind the Success
This breakthrough builds on decades of research. The work utilizes research from Harvard's Melton Lab, where scientists have been working on converting stem cells into insulin-producing cells.
What makes this approach special is that these aren't just any cells that make insulin. They're fully developed islet cells that can respond to blood sugar changes just like your natural pancreas would. It's the difference between having a skilled chef who can adjust a recipe on the fly versus someone who can only follow a basic recipe.
Treatment Comparison
Daily Insulin Injections:
Multiple injections daily
Constant monitoring
Risk of highs/lows
Zimislecel Treatment:
One-time infusion
Natural insulin production
83% insulin independence
What's Next: The Road to Approval
So when might this treatment become available to patients? The timeline is encouraging but still requires patience.
Vertex Pharmaceuticals is currently enrolling patients in the Phase III arm of the trial across the U.S., Canada and Europe. The company expects to initiate regulatory submissions for approval next year.
This means we're not talking about decades of waiting. We're potentially looking at a few years before this treatment could become available to patients who need it most.
Evolution of diabetes management technology and treatment approaches
Beyond the Numbers: Real Impact on Lives
Statistics tell one story, but the real impact is in the lives changed. Imagine a teenager who can go to sleepovers without worrying about nighttime blood sugar crashes. Picture a parent who doesn't have to wake up every night to check their child's glucose levels.
This treatment represents more than medical progress. It's about giving people their freedom back. It's about turning a lifelong medical condition into a manageable chapter in someone's story.
The Bigger Picture: Hope for Diabetes Treatment
This breakthrough is part of a larger revolution in diabetes care. Recent advances include successful stem cell therapies in China, where a 25-year-old woman became the first person to receive insulin-producing cells from her own reprogrammed stem cells.
Why does this matter? Because it shows that multiple approaches are working. Scientists around the world are finding different ways to solve the same problem. It's like having multiple teams working on the same puzzle from different angles.
"The magnitude, consistency and durability of the results from all 12 patients with more than one year of follow-up reinforce the transformative potential of zimislecel for people living with type 1 diabetes complicated by severe hypoglycemia."
- Dr. Carmen Bozic, Vertex Pharmaceuticals
Looking Forward: Questions and Considerations
While these results are exciting, important questions remain. How long will the treatment effects last? Can the approach be refined to work without immunosuppressive drugs? Will it work for all types of Type 1 diabetes patients?
These are the questions that researchers are working to answer. Each study, each patient, each success brings us closer to understanding how to make this treatment even better.
The Human Element
Behind every percentage, every clinical trial participant, every data point is a real person with hopes, fears, and dreams. This treatment isn't just about restoring insulin production. It's about restoring hope.
For the diabetes community, this represents a shift from managing a condition to potentially curing it. That's not just medical progress – that's life-changing progress.
Key Takeaways

Final Thoughts
The journey from insulin injections to insulin independence is becoming a reality for some patients. While zimislecel isn't a cure-all for every person with Type 1 diabetes, it represents hope for those who need it most.
This breakthrough reminds us that medical science doesn't just treat diseases – it transforms lives. For the 83% of patients who achieved insulin independence, the future looks very different than it did just a few years ago.
As we wait for broader availability, this treatment stands as proof that the impossible can become possible. Sometimes, the best medicine isn't just what heals the body – it's what restores hope for the future.
##############
Feel Free to share this content article with a colleague or friend who need to learn about this! 📧
References:
Vertex Pharmaceuticals - Type 1 Diabetes Pipeline
New England Journal of Medicine - Clinical Trial Results
Inside Precision Medicine - Original Article
Harvard Stem Cell Institute - Melton Lab Research
Clinical Trial Database - FORWARD-101 Study
Disclaimer: This article is for informational purposes only and should not replace professional medical advice. Patients interested in cell therapy treatments should consult with their healthcare providers about their specific situation and treatment options.